thunderstruck
perchlorate pollution
from fireworks
February 12, 2011
thunderstruck
perchlorate pollution
from fireworks
February 12, 2011
Welcome to BadwaterJournal.com© INDEX PAGE for all articles
Looking south from the Second Street Bridge to Louisville, Kentucky, at the Ohio River, site of the largest outdoor fireworks display in America, and largest fireworks perchlorate release.
EPA is preparing to regulate perchlorate levels in drinking water. Louisville is exploding 60 tons of perchlorate containing fireworks over the Ohio.

Michael E. Berry, President William Douglas Taylor, President
Kentucky Derby Festival, Inc. Zambelli Fireworks Manufacturing Co.
1001 South Third Street PO Box 1463
Louisville, KY 40203 New Castle, PA 16103-37
DEP Division of Water United States EPA Region 4
200 Fair Oaks Lane Sam Nunn Atlanta Federal Center
Fourth Floor 61 Forsyth Street, SW
Frankfort, KY 40601 Atlanta, GA 30303-8960
March 1, 2011
Dear Sirs,
This is a request for information about contamination, pollution and public health risk caused by perchlorate and metals releases from pyrotechnic displays at the Thunder Over Louisville now scheduled for April 16th in Louisville, Kentucky.
I am a resident of Louisville in the area affected by contamination of air, soil, groundwater and surface water from the release of pollutants from combustion of 60 tons of fireworks. State and federal laws prohibit pollution of air and water unless in compliance with a valid permit. The Ohio River is a drinking water source for millions of people.
1.Have you applied for and received any air or water pollution discharge permit for Thunder Over Louisville?
2.Have you performed or contracted for any air or water quality monitoring to detect resulting concentrations of perchlorate and metals: barium, strontium, copper, aluminum caused by the release of 60 tons of fireworks on April 16th, 2011 ?
3.Perchlorate is released by fireworks combustion and contaminates drinking water. Perchlorate interferes with thyroid function and hormone production and poses a public health risk. Have you obtained any expert opinion regarding the public health risk of Thunder Over Louisville on the 16th of April? Have you identified any population subgroups that should not attend the pyrotechnics display due to polluted aerosols?
4.Have you identified downstream drinking water intakes affected by the release and the fate of perchlorate in fish tissues that may enter the food chain?
Thank You in advance for your information. This information will be published on the blog site http://badwaterjournal.com
Clarence Hixson
Attorney at Law
1336 Hepburn Avenue
Louisville, KY 40204
(502)758-0936
Cc: Kentucky Division of Water, Metro Air Pollution Control Board, USEPA






The 2nd Street Bridge has a combined sewer overflow on the Indiana side under the bridge. Debris from high water covers the river bank with a dazzling array of consumer cast off trash.

How many tons of perchlorate will be released to air and water?
Where are the Health Department and air and water pollution regulators?
Perchlorate is both a man-made and naturally occurring chemical. It is used in rocket fuel, explosives, fireworks, and other products. Naturally occurring perchlorate is produced through atmospheric processes and then settles on surface water or land. Perchlorate can disrupt the uptake of iodide in the thyroid, potentially interfering with thyroid function and negatively affecting fetal and infant brain development and growth. As of June 2010, there is no federal regulatory standard for perchlorate in drinking water, and the Environmental Protection Agency (EPA), which has the authority to regulate contaminants in public drinking water systems, had not determined whether to establish one.
EPA has announced it will regulate perchlorate in drinking water
Final Regulatory Determination on Perchlorate issued February 2011
Perchlorate has been found in water and other media at varying levels in 45 states, as well as in the food supply, and comes from a variety of sources. EPA conducted one nationwide perchlorate sampling, between 2001 and 2005, and detected perchlorate at or above 4 parts per billion in 160 of the 3,865 public water systems tested (about 4.1 percent).
In 31 of these 160 systems, perchlorate was found above 15 parts per billion, EPA's current interim health advisory level. Sampling by DOD, NASA, and DOE detected perchlorate in drinking water, groundwater, surface water, soil, and sediment at some facilities.
For example, GAO's analysis of DOD data showed that perchlorate was detected at almost 70 percent of the 407 installations sampled from fiscal years 1997 through 2009, with detections ranging from less than 1 part per billion to 2.6 million parts per billion.
A 2006 Food and Drug Administration study found perchlorate in 74 percent of 285 food items tested, with certain foods, such as tomatoes and spinach, having higher perchlorate levels than others. According to researchers, concentrations of perchlorate at or above 100 parts per billion generally result from activities involving man-made perchlorate, such as the use of perchlorate as a rocket propellant.
California and Massachusetts have adopted their own standards. California adopted a drinking water standard of 6 parts per billion in 2007, and Massachusetts set a drinking water standard of 2 parts per billion in 2006.
http://www.gao.gov/mobile/products/GAO-10-769
Perchlorate: Occurrence Is Widespread but at Varying Levels; Federal Agencies Have Taken Some Actions to Respond to and Lessen Releases
GAO-10-769, August 12, 2010
Highlights Page (PDF) Full Report (PDF, 63 pages) Accessible Text
Ammonium chlorate and potassium chlorate are the primary oxidizers added to fireworks formulations to make the big bangs and bright colors.
Perchlorate released in water may enter drinking water intakes downstream and be supplied to cattle and contaminate milk supplies.
“Perchlorate disrupts thyroid function by competitively inhibiting iodide transport (2). The resulting malfunction of the Na+-I- Symporter (NIS) (3) reduces thyroid hormone production (4) and can impair the development of the gland. Pregnant women, children, and people with compromised thyroid function are thus particularly at risk.”
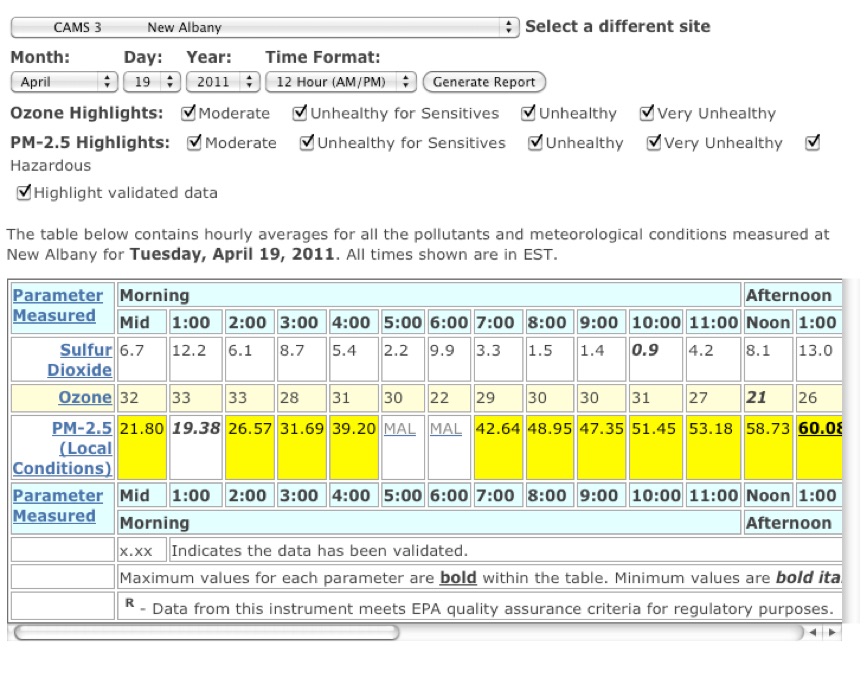
Day after Thunder air pollution monitoring results from the New Albany Indiana air monitoring station shown below. The pollutant is fine particulate pollution showing multiple hour violations of the 35 micro gram per cubic meter National Ambient Air Quality Standards---yellow boxes. The IDEM website was
Some percentage of an estimated 3 tons of perchlorate will be released to air and water at Thunder Over Louisville.
Fantastic super jets and helicopters scream overhead, families stroll and mingle in the enormous crowd along the Louisville waterfront, breathless TV talking heads describe the awesome lethal power of the military aircraft--Thunder Over Louisville provides bread and circuses for the restless citizens while ginning up adulation for warriors. Its not an event where people reflect soberly on the tragedy of modern industrial and robotic warfare as practiced against stone age villagers in distant countries.
If you have been there, you know that sometimes thick and pungent clouds of smoke from the tons of fireworks rolls through the crowds, making eyes water and people cough. Whats in the smoke? and whats left in the water after the big celebration?
Kentucky Derby Festival website
<http://kdf.org/events-Thunder-Over-Louisville.html>
“The show will feature the latest in pyrotechnic power from Zambelli Internationale, America's "first family of fireworks". Eight 400-foot barges assemble on both sides of the 2nd Street Bridge to form the stage from which the fireworks spectacular ignites.
The breathtaking and mind-numbing 28-minute show includes the Zambelli's signature one-mile "waterfall" effect off the bridge, making the fireworks seem to rain down forever. And in the crowd's memory of the show, it does!
After more than a decade, the show remains the largest annual pyrotechnic display in North America. Seen by millions worldwide via a July 4th rebroadcast on the Armed Forces Network to more than 150 countries, it is the state's pride.”
To assemble a show the size of Thunder, Zambelli Internationale utilizes eight tractor trailers filled with nearly 60 tons of fireworks shells. The physical set-up includes 250 tons of launching tubes, some as high as 10 feet with a diameter of 1 and 1/2 feet. Two million pounds of sand will pack the firing tubes on 1,800 feet of barges. Almost 700 miles of wire cable connecting 20 firing boards are tied to the command post for synchronization to the music. This is the largest show the Zambellis perform each year and the Zambellis are the largest fireworks family in the world.
<http://thunderoverlouisville.org/the-fireworks.html>
http://www.gao.gov/mobile/products/GAO-10-769
“ The report fails to mention Department of Commerce data, provided to GAO by DoD, describing the large amount of perchlorate imported into the U.S. primarily for fireworks and used in an uncontrolled manner over large areas. This data provides perspective on DoD's use versus imports for commercial use. According to the American Pyrotechnics Association, 278.2 million pounds of fireworks were consumed in the U.S. annually over a recent 5 year period, approximately 14 million pounds of which is perchlorate.”
Assistant Secretary of Defense, John Conger in a letter to the Government Accountability Office dated August, 2010, in response to the GAO Report on military use and soil and water contamination by perchlorate.
Interim Drinking Water Health Advisory For Perchlorate
EPA 822-R-08-025
December 2008
Two states have established regulatory standards for perchlorate in drinking water. In July 2006, Massachusetts promulgated a drinking water standard of 2 μg/L for perchlorate (Mass DEP, 2006), while California established an MCL of 6 μg/L in October 2007 (CDPH, 2007).
The states used the same NOEL from the Greer et al. (2002) study as EPA, but different methodologies for calculating RSC and addressing sensitive subpopulations. EPA believes that its analysis is based on the best currently available science, data, and analyses, including some analyses that were not available at the time these state standards were established, and that drinking water with perchlorate concentrations at or below its interim HA level of 15 μg/L is protective of all subpopulations.
The SDWA allows States to establish drinking water standards that are more stringent than EPA’s national standards, as well as for contaminants which EPA has determined not to regulate. EPA supports such state action, especially in cases where there are not enough public water systems above the interim HA level to warrant a national standard, but where a few individual states may have a higher concentration of such systems.
Office of Water (4607M) EPA 815-F-11-003 February 2011 water.epa.gov/drink
“Why did EPA decide to regulate perchlorate?
EPA has determined that perchlorate meets Safe Drinking Water Act’s (SDWA’s) three criteria for regulating a contaminant.
1)Perchlorate may have adverse health effects because scientific research indicates that perchlorate can disrupt the thyroid’s ability to produce hormones needed for normal growth and development.
2)There is a substantial likelihood that perchlorate occurs with frequency at levels of health concern in public water systems because monitoring data show over four percent of public water systems have detected perchlorate, and
3) There is a meaningful opportunity for health risk reduction for the between 5.2 and 16.6 million people who may be served drinking water containing perchlorate.”
For information on perchlorate, please visit
http://water.epa.gov/drink/contaminants/unregulated/perchlorate.cfm.
Louisville’s skyline and politics reflect the influence of eccentric personalities--not unexpected for the home of bourbon whiskey magnates, fried chicken empires, baseball bat makers, and horse racers. Perhaps giant firecracker statues should be distributed around the city, like the Thoroughbred Horse figures that have become ubiquitous at trendy shops.




_________________________________
Effect of fireworks events on urban background trace metal aerosol concentrations:
Is the cocktail worth the show?
Teresa Morenoa, Xavier Querola, Andrés Alastueya, Fulvio Amatoa, Jorge Peya, Marco Pandolfia, Nino Kuenzlib, Laura Bousoc, Marcela Riverac and Wes Gibbonsd
a Institute of Environmental Assessment and Water Research, IDAEA, CSIC, C/Jordi Girona 18, Barcelona 08034, Spain
b Institute for Social and Preventive Medicine at Swiss Tropical Institute Basel, Steinengraben 49, 4051 Basel, Switzerland
c CREAL – Center for Research in Environmental Epidemiology, Doctor Aiguader 88, 08003 Barcelona, Spain
We report on the effect of a major fireworks event on urban background atmospheric Particulate Matter 2.5 chemistry, using 24-h data collected over 8 weeks at two sites in Girona, Spain.
The firework pollution episode (Sant Joan fiesta on 23rd June 2008) measured in city centre parkland increased local background Particulate Matter 2.5 concentrations as follows:
Strontium (x 86),
Potassium (x 26),
Barium (x 11),
Cobalt (x 9),
Lead (x 7),
Copper (x 5),
Zinc (x 4),
Bismuth (x 4),
Magnesium (x 4),
Rb (x4),
Sb (x3),
P (x3),
Ga (x2),
Mn (x2),
As (x2),
i (x2) and
SO42− (x2).
Marked increases in these elements were also measured outside the park as the pollution cloud drifted over the city centre, and levels of some metals remained elevated above background for days after the event as a reservoir of metalliferous dust persisted within the urban area.
Transient high-Particulate Matter pollution episodes are a proven health hazard, made worse in the case of firework combustion because many of the elements released are both toxic and finely respirable, and because displays commonly take place in an already polluted urban atmosphere.
_________________________________
Heavy metals from pyrotechnics in New Years Eve snow
Georg Steinhauser, Johannes H. Sterbaa, Michaela Fostera, Friedrich Grassa and Max Bichlera
aVienna University of Technology, Atominstitut der Österreichischen Universitäten, Stadionallee 2, 1020 Wien, Austria
Abstract
Pyrotechnics and fireworks cause pollution with barium aerosols, which is a result of the utilization of barium nitrate as a combined pyrotechnic oxidizer and coloring agent. In this study, the washing-out of barium-rich aerosols by snowflakes during the New Years Eve celebrations in an Austrian village in the Alps has been investigated. It could be shown that the fireworks caused an increase in the barium concentration in snow of up to a factor of 580 compared to the blank value. An increase of the concentrations of strontium and occasionally arsenic in snow was also observed. The geographic distribution of the pyrotechnic combustion products on this snowy evening was restricted to a relatively small area and even in a very local scale, the variations in the concentrations were remarkable. Post-firework snow from the summits of nearby located mountains was found to be as clean as pre-firework snow. However, snow that was visibly contaminated with smoke residues contained exorbitant concentrations of Ba, K, Sr, and Fe.
_______________________________
Dartmouth, Massachusetts
August 2007
Massachusetts Department of Environmental Protection
1 Winter Street Boston, MA 02108
<http://www.mass.gov/dep/cleanup/sites/umdrep.htm>
FINAL REPORT:
EVALUATION OF PERCHLORATE CONTAMINATION AT A FIREWORKS DISPLAY
“Based on field investigations conducted since 2004, it appears that 11 years of fireworks displays have resulted in perchlorate contamination in soil and groundwater at the Perchlorate Study Area. These conclusions are consistent with fate and transport modeling results for this area.”
________________________________________________________________
Environ. Sci. Technol. 2007, 41, 3966-3971
Perchlorate Behavior in a Municipal Lake Following Fireworks Displays
R I C H A R D T . W I L K I N , * , †
D E N N I S D . F I N E , ‡ A N D
N I C O L E G . B U R N E T T
“Perchlorate salts of potassium and ammonium are the primary oxidants in pyrotechnic mixtures, yet insufficient information is available regarding the relationship between fireworks displays and the environmental occurrence of perchlorate.”
“Preceding fireworks displays, perchlorate concentrations in surface water ranged from 0.005 to 0.081 μg/L, with a mean value of 0.043 μg/L. Within 14 h after the fireworks, perchlorate concentrations
spiked to values ranging from 24 to 1028 times the mean baseline value. A maximum perchlorate concentration of 44.2 μg/L was determined following the July 4th event in 2006.”
________________________________________________________________
http://www.startribune.com/local/49978972.html
“The fireworks were fun, but they came at a price.”
“Particle pollution in the Twin Cities was elevated Sunday because of Saturday night's fireworks all over the metro area, the Minnesota Pollution Control Agency said Sunday.
The agency issued an "orange" notification after light winds failed to disperse the pollutants that lingered after the celebratory fanfare and fumes. People with asthma and other health problems, as well as elderly people, were advised to stay indoors, and everyone was urged to avoid prolonged outside exertion.”
“By today, the Twin Cities air pollution level should be down to "moderate," where it is expected to remain through this week, the agency said. With a "moderate" designation, sensitive groups should continue to avoid outdoor exertion, the agency said.”
_____________________________________________________________
The significant occurrence of perchlorate in all milk samples analyzed at levels that are comparable or even greater than the current California “action level” for the concentration of perchlorate in drinking water came as a considerable surprise to us.
Environ. Sci. Technol. 2003, 37, 4979-4981
Perchlorate in Milk
A N D R E A B . K I R K , † E R N E S T E . S M I T H , * , †
K A N G T I A N , † T O D D A . A N D E R S O N , † A N D
P U R N E N D U K . D A S G U P T A * , † , ‡
Perchlorate was unambiguously detected by ion chromatography-suppressed conductivity (IC-CD) and/or ion chromatography-electrospray mass spectrometry (IC-MS) in seven of seven supermarket milk samples bought randomly in Lubbock, TX. Quantitation by IC-MS and ICsuppressed conductivity detection in conjunction with a preconcentration-preelution method provided comparable results. With a sample cleanup procedure that involved protein removal by ethanol and sequential passage though activated alumina and C-18 silica, the limit of detection for perchlorate in milk was 0.5 μg/L. The levels found ranged from 1.7 to 6.4 μg/L. An evaporated milk sample contained perchlorate at 1.1 ( 0.6 μg/L level, while we did not find detectable levels in a reconstituted powdered milk sample.
In recent years the concern about perchlorate in drinking water has become such a major public issue that lengthy articles have appeared in the Wall Street Journal (1), even as a front page story. Perchlorate disrupts thyroid function by competitively inhibiting iodide transport (2). The resulting malfunction of the Na+-I- Symporter (NIS) (3) reduces thyroid hormone production (4) and can impair the development of the gland. Pregnant women, children, and people with compromised thyroid function are thus particularly at risk. Although the EPA is yet to set a specific drinking water limit, the State of California has already adopted an action level of 4 μg/L (5) based on the draft toxicology and risk assessment review (6).




_______________________________
Environ. Sci. Technol. 2010, 44, 8295–8301
Particulate Oxidative Burden
Associated with Firework Activity
K R Y S T A L J . G O D R I , † , ‡ D A V I D C . G R E E N , †
G A R Y W . F U L L E R , † M A N U E L D A L L ’ O S T O , ‡
D A V I D C . B E D D O W S , ‡ F R A N K J . K E L L Y , * , †
R O Y M . H A R R I S O N , ‡ A N D
I A N S . MUDWAY†
“Despite the extensive use of fireworks to commemorate special events internationally, no literature has yet described the toxicity associated with firework PM on the respiratory system.”
Firework events are capable of inducing particulate matter (PM) episodes that lead to exceedances of regulatory limit values. Short-term peaks in ambient PM concentration have
been associated with negative impacts on respiratory and cardiovascular health
Pyrotechnic combustion events were characterized by increased gas phase pollutants levels (NOx
and SO2), elevated PM mass concentrations, and trace metal concentrations (specifically Sr, Mg, K, Ba, and Pb). Relationships between NOx, benzene, and PM10 were used to apportion firework and traffic source fractions. A positive significant relationship was found between PM oxidative burden and
individual trace metals associated with each of these apportioned
source fractions. The level of exposure to each source fraction was significantly associated with the total OP. The firework contribution to PM total OP, on a unit mass basis, was greater than that associated with traffic sources: a 1 μg elevation in firework and traffic PM fraction concentration
was associated with a 6.5 ( 1.5 OPT μg/L and 5.2 ( 1.4 OPT μg/L increase, respectively. In the case of glutathione depletion, firework particulate OP (3.5 ( 0.8 OPGSH μg/L) considerably exceeded that due to traffic particles (2.2 ( 0.8 OPGSH μg/L).
Therefore, in light of the elevated PM concentrations caused by firework activity and the increased oxidative activity of this PM source, there is value in examining if firework derived PM is related to acute respiratory outcomes.
_______________________________
Environ. Sci. Technol. 2006, 40, 6608–6614
Perchlorate in the United States.
Analysis of Relative Source
Contributions to the Food Chain
PERNENDU K. DASGUPTA et al.
“[O]ver this 30 year period [1976 -2005] fireworks consumption has been rising exponentially . . .
the average consumption over the last thirty years can be estimated to be 4.5 x 107 kilograms per year.
Fireworks vary greatly in their type and composition and perchlorate content. Some components can contain up to 70% ammonium perchlorate or Potassium perchlorate, and there are fireworks that contain very little perchlorate or no perchlorate. Presently available information does not allow us to determine the total amount of perchlorate present in fireworks consumed in the U.S. and how much of that enters the environment in an unburned or partially burned state. Because of the paucity of information, we cannot consider the impact of fireworks quantitatively at this time. However, the fact that U.S. consumption of fireworks is exponentially rising and that such displays are often carried out in the vicinity of large water bodies strongly suggest a potential for significant contamination that must be quantitatively assessed in the future.”
_________________________________
LETTER REQUESTING AIR/WATER MONITORING
Dr. John B. Stephenson
Director Natural Resources and Environment
Dr. Richard T Wilkin
National Risk Management Research Laboratory
USEPA
Dr. Pernendu K. Dasgupta
Dept of Chemistry
Texas Tech University
Dear Sirs,
I have read your articles and Reports , "Perchlorate in the United States. Analysis of Relative Source Contributions to the Food Chain." Environ. Sci. Technol. 2006, 40, 6608-6614
and
"Perchlorate Behavior in a Municipal Lake Following Fireworks Displays" Environ. Sci. Technol. 2007, 41, 3966-3971
and
GAO-10-769, August 2010,
PERCHLORATE : Occurrence Is Widespread but at Varying Levels;
Federal Agencies Have Taken Some Actions to Respond to and Lessen Releases
Kentucky provided no percolate release data for the GAO Report. In this case the largest pyrotechnics display in North America will be held on April 16, 2011 on the river at Louisville Kentucky. The constituents of 60 tons of fireworks from 8 semi trailers will be exploded over a major source of drinking water and in the midst of a crowd of spectators including unwarned pregnant women.
I have sent a letter to the ORSANCO water association recommending water quality monitoring for perchlorate, before during and after the display, but received no answer. This event on the 16th provides a uniques opportunity to capture data that focuses on fireworks contribution to air and water pollution and useful for identifying public health concerns.
I urge you to explore your contacts among peers and help me make sure that this event does not
pass without thorough scientific documentation. I appreciate any efforts toward this goal.
Clarence H. Hixson
1336 Hepburn Avenue
Louisville, KY 40204
(502) 758-0936
See,
Welcome to BadwaterJournal.com© INDEX PAGE for all articles
